Infiltration and contact angle
The phenomenon of lotus roots being pulled out of mud is relatively clean, the water on the surface of lotus leaves is approximately spherical, water striders can walk on the water surface, raincoats can prevent rain, and pesticide solutions can be dispersed on the surface of crops, which is related to the wetting of solid surfaces by liquids (also known as wetting). The phenomenon of wetting is caused by the surface tension of intermolecular interactions, which is the free energy per unit area at the interface. Figure 1-1 shows the static wetting model of liquid on a solid surface:
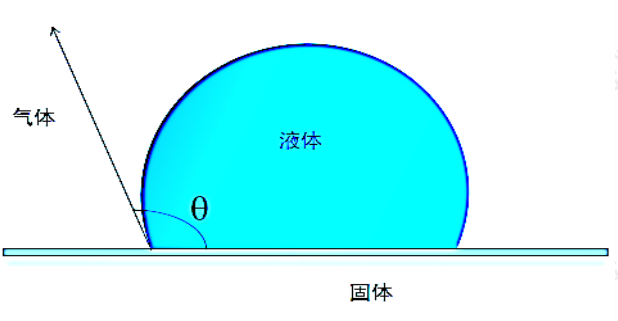
Figure 1-1 Schematic diagram of static contact angle
contact angle θ It refers to the angle between the liquid solid interface and the gas liquid interface at the gas liquid solid interface (triple line, also known as the contact point), used to measure the degree of infiltration. The smaller the value, the better the infiltration. Assuming that it is not limited by the molecular diameter, the liquid spreads infinitely on the solid surface, which is called complete wetting. At this point θ= 0 °; The contact surface between liquid and solid has a certain area and 0 °< θ< 180 ° is called incomplete infiltration; If θ= 180 ° is called complete non infiltration. General definition θ< At 90 °, the liquid can moisten (saturate) the solid; θ> At 90 °, liquids cannot wet solids.
Water is the most common liquid, and its infiltration corresponds to hydrophobicity. The larger the contact angle, the better the hydrophobicity.
● When θ< At 90 °, the corresponding material is usually referred to as hydrophilic;
● When θ> At 90 °, the corresponding material is usually referred to as hydrophobic.
The wetting effect of liquid on solid can generally be characterized by adhesion work, which represents the work required to separate the interface between liquid and solid per unit area in vacuum, leaving behind the bare solid surface, i.e
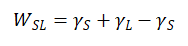
(1-1)
In the formula, is the adhesion work, is the surface tension of solid and liquid in vacuum, is the interfacial tension between liquid and solid.
The greater the adhesion work, the better the wettability of the liquid. Although adhesion work can accurately characterize wettability, in practice, due to the involvement of surface tension of solids, accurate measurement is difficult, and it is difficult to achieve the method of using adhesion work to characterize wettability. In order to obtain a quantity that is convenient for practical measurement and can accurately characterize wettability, it was found through deduction that the adhesion work satisfies the following relationship with the surface tension and contact angle of the liquid
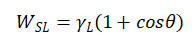
(1-2)
Equation (1-2) is called the Young Drupe formula, which provides the relationship between adhesion work, liquid surface tension, and contact angle. Assuming a contact angle of 180 °, it can be inferred from equations (1-2) that the adhesion work is 0, there is no attraction between the solid and liquid, and no work is required during the separation process. The liquid cannot completely infiltrate the solid.
Substitute equation (1-1) into equation (1-2) to obtain equation (1-3)
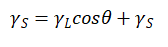
(1-3)
Equations (1-3) are called the Young equation, θ Also known as the Young contact angle.
PREV:Common measurement methods for static contact angle
NEXT:Application of contact angle meter in wettability analysis of carbon materials

